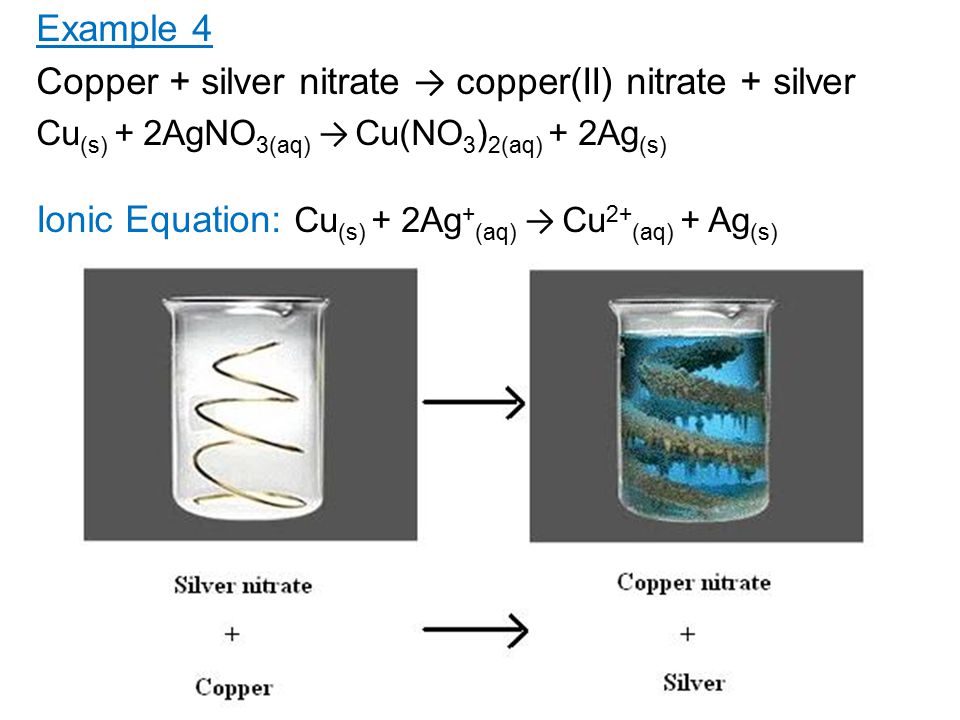
Observe the diagram showing a copper rod kept immersed in silver nitrate solution.a. What is the colour change of the solution?b. Write the balanced chemical equation for the reaction.
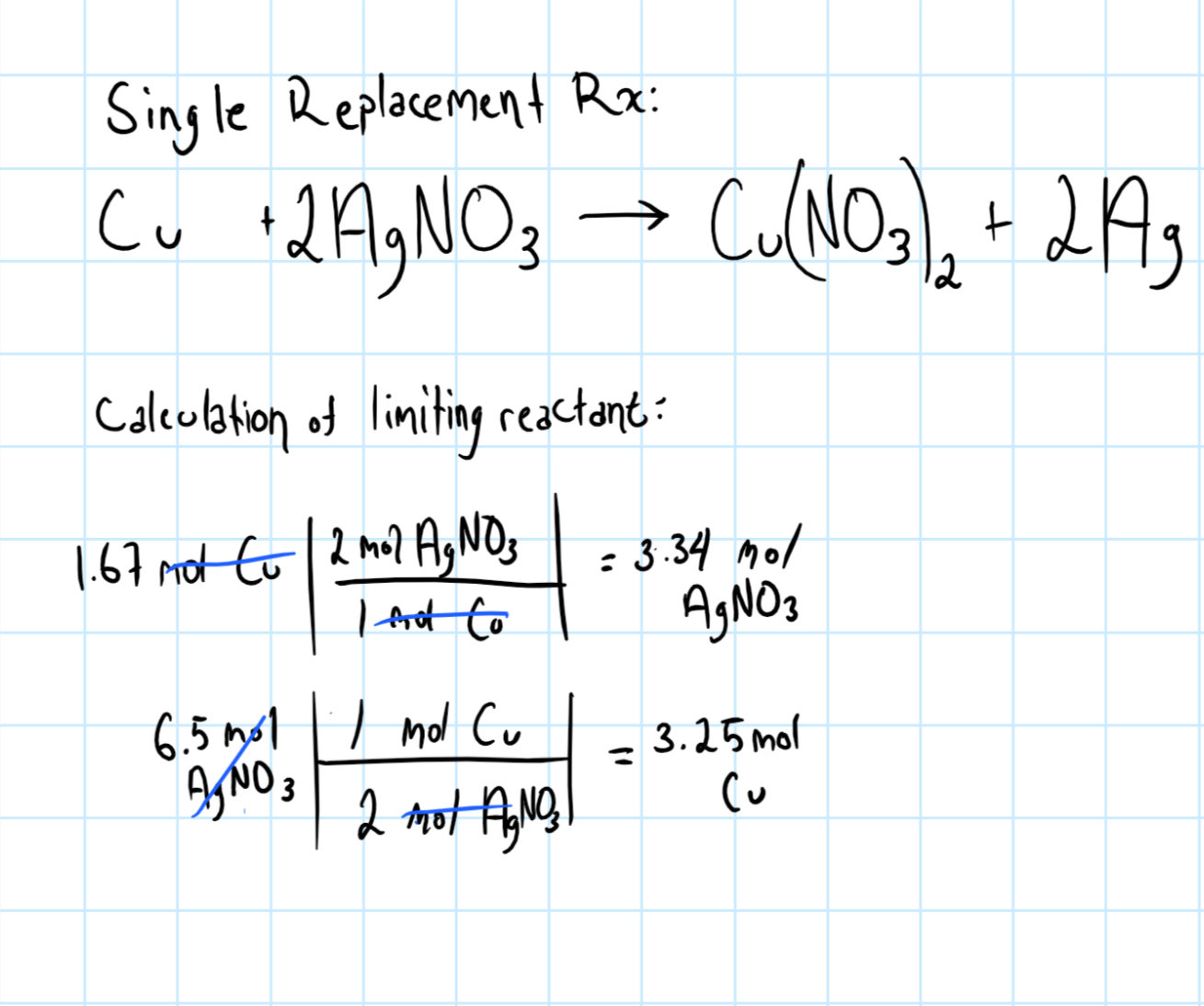
If 1.67 mol of copper and 6.5 mol of silver nitrate are available to react by single replacement, what is the limiting reactant? | Socratic
If 34.5 g of copper reacts with 70.2 g of silver nitrate, according to the following reaction, what is the maximum number of grams of silver that can be produced? - Quora

STOCK IMAGE, , JB7956, 01B466TS , Science Source - Search Medical & Scientific Stock Photos at MedicalImages.com

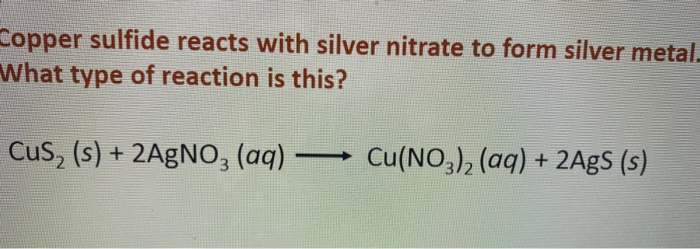
Copper wire reacts with silver nitrate to form silver and copper (1) nitrate. This reaction is - Brainly.com
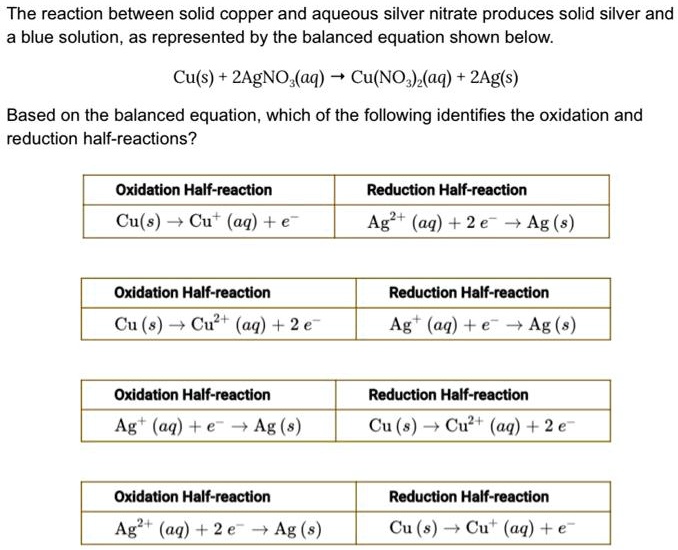
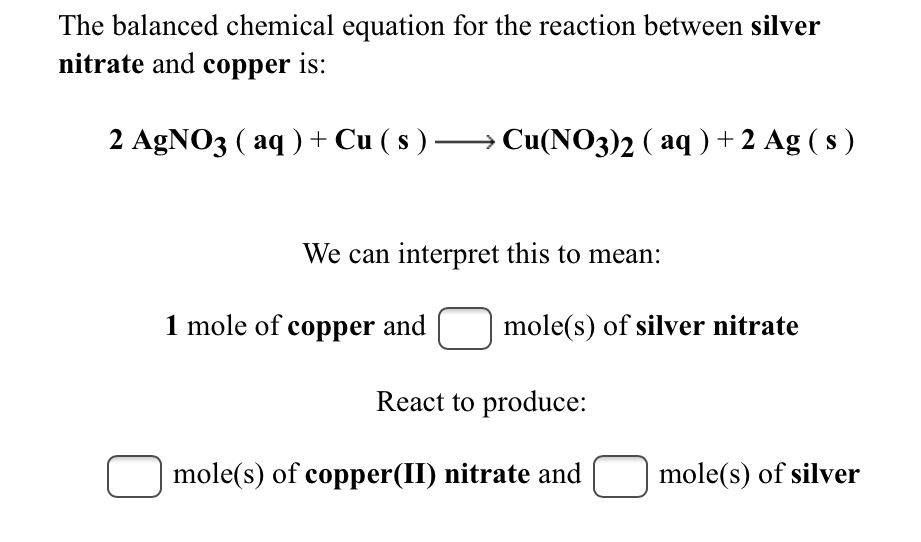
SOLVED: The reaction between solid copper and aqueous silver nitrate produces solid silver and blue solution, as represented by the balanced equation shown below: Cu(s) 2AgNO (aq) Cu(NO )(aq) 2Ag(s) Based on



/copper-wire-immersed-in-silver-nitrate-causing-blue-colour-81991997-582f14595f9b58d5b1a9b484.jpg)