On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

Early-drug development in the era of immuno-oncology: are we ready to face the challenges? - Annals of Oncology

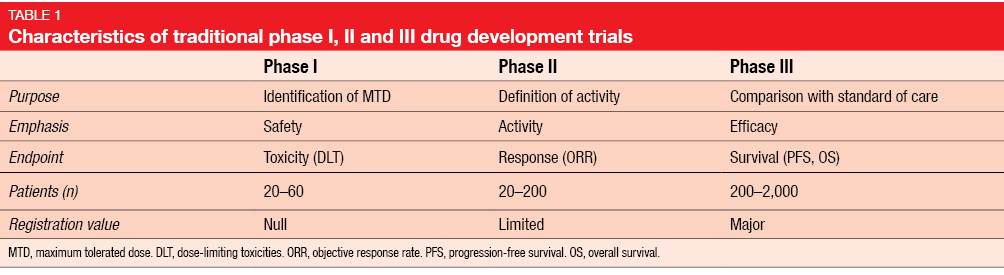
Challenges of phase 1 clinical trials evaluating immune checkpoint-targeted antibodies - Annals of Oncology

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram

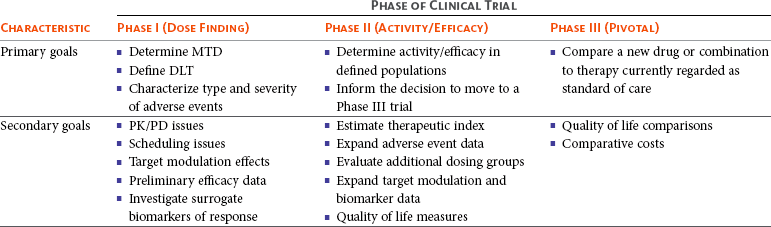
Designs of drug-combination phase I trials in oncology: a systematic review of the literature - Annals of Oncology

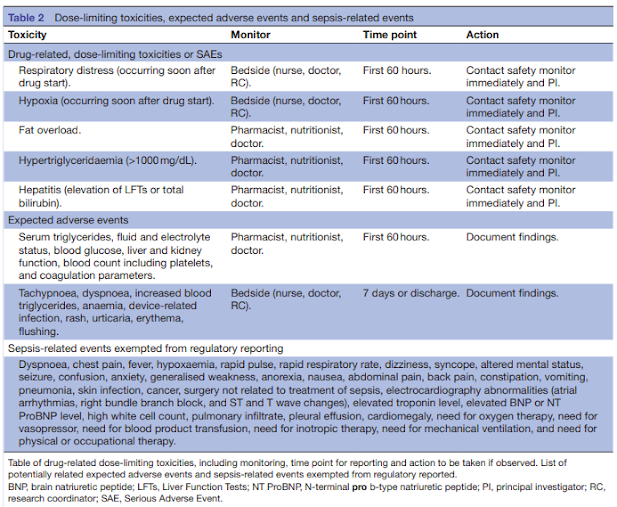
Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group
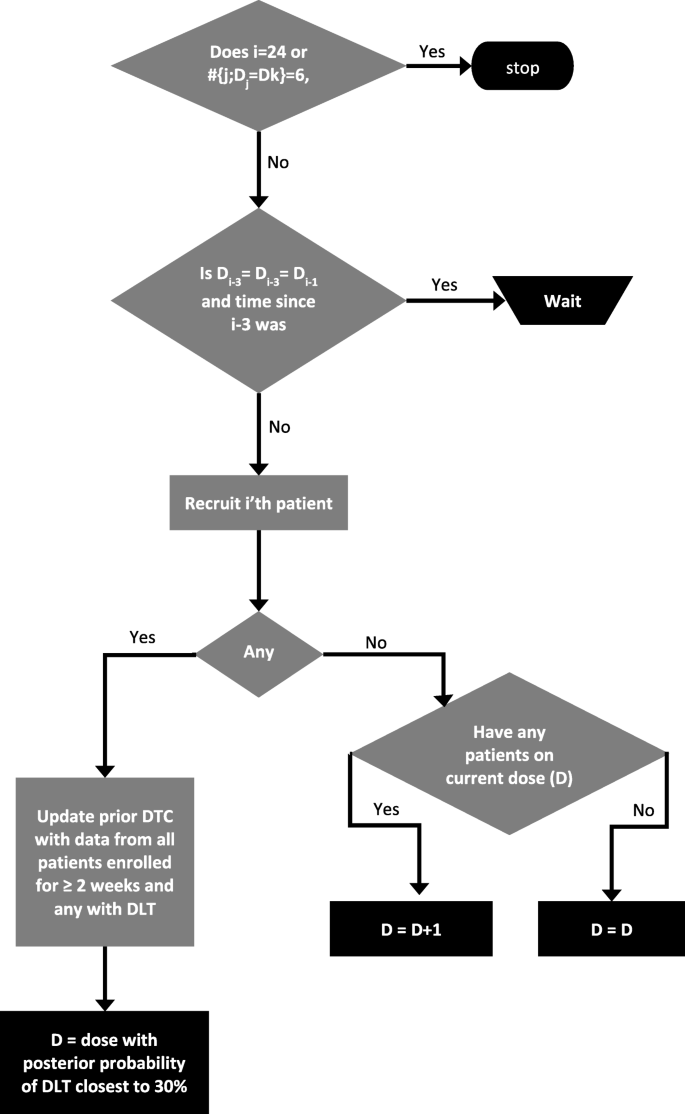
Dose Limiting Toxicities (DLT) DOSE LIMITING TOXICITIES (DLT) Example: Dose escalation will proceed within each cohort according
Guidance for Industry Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment

Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library
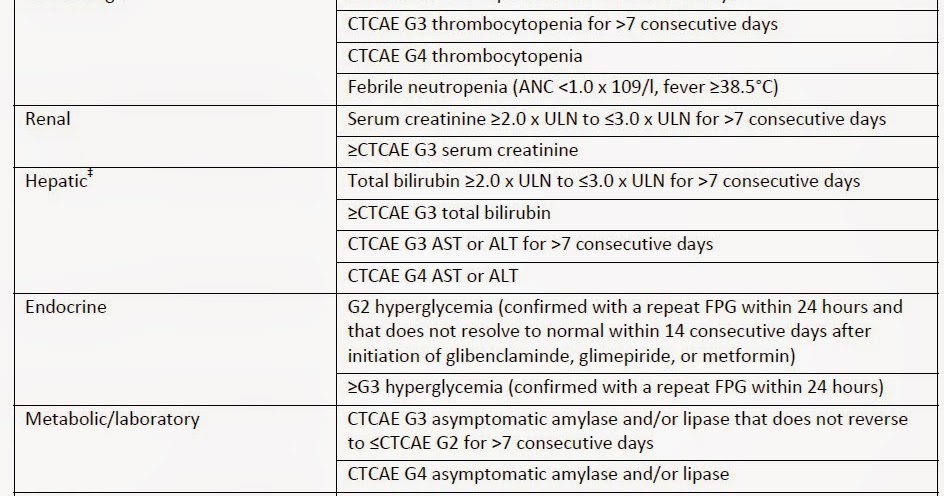
On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)