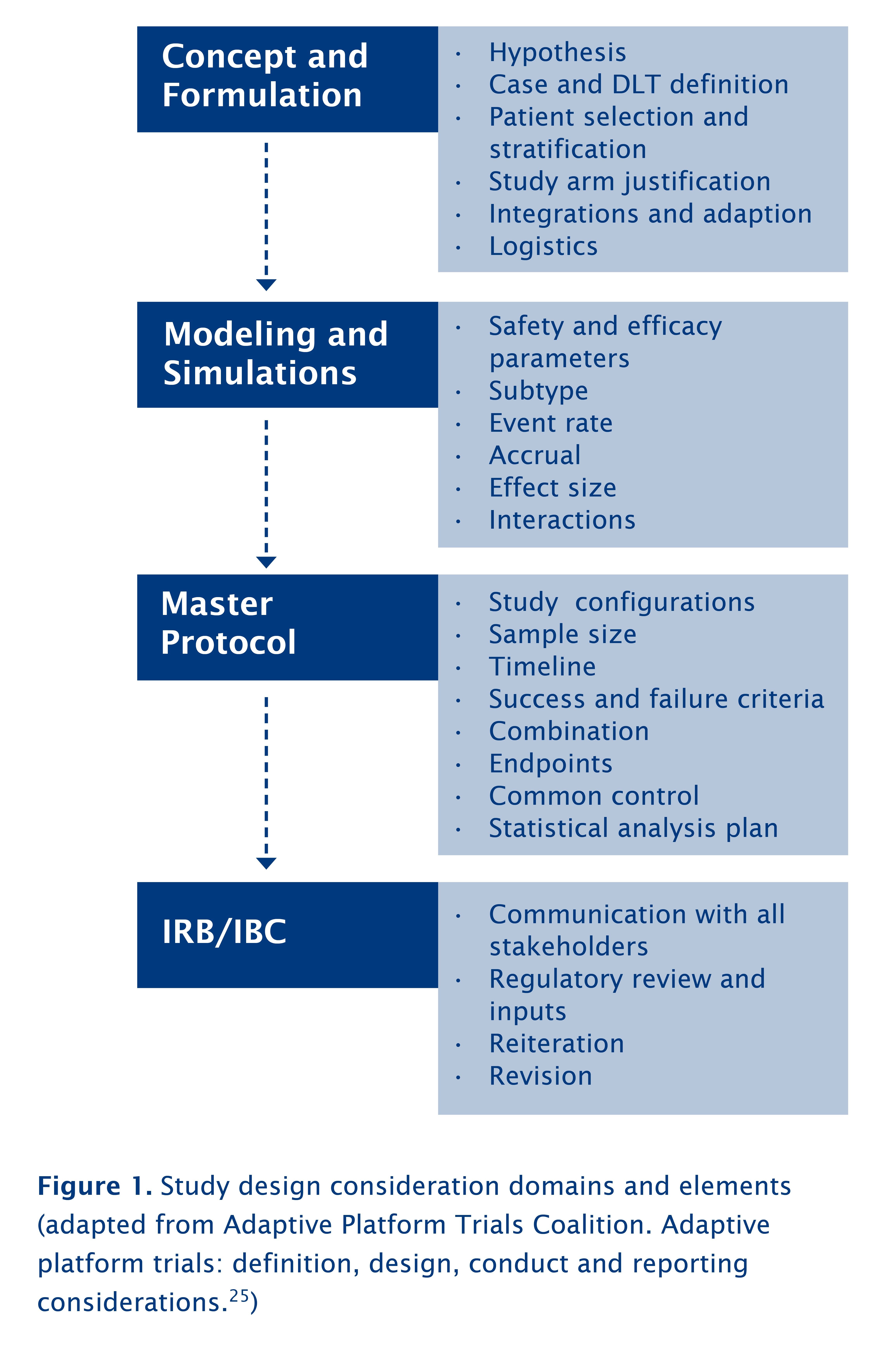
Advanced Methods for Dose and Regimen Finding During Drug Development: Summary of the EMA/EFPIA Workshop on Dose Finding (London 4–5 December 2014) - Musuamba - 2017 - CPT: Pharmacometrics & Systems Pharmacology - Wiley Online Library
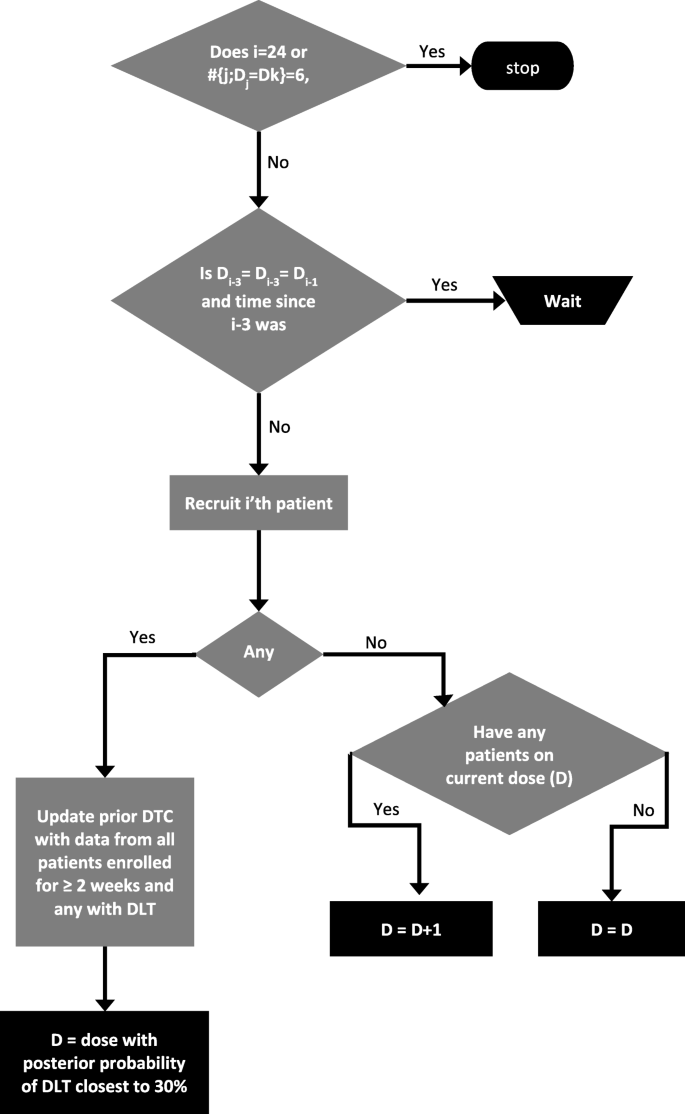
A new pragmatic design for dose escalation in phase 1 clinical trials using an adaptive continual reassessment method | BMC Cancer | Full Text
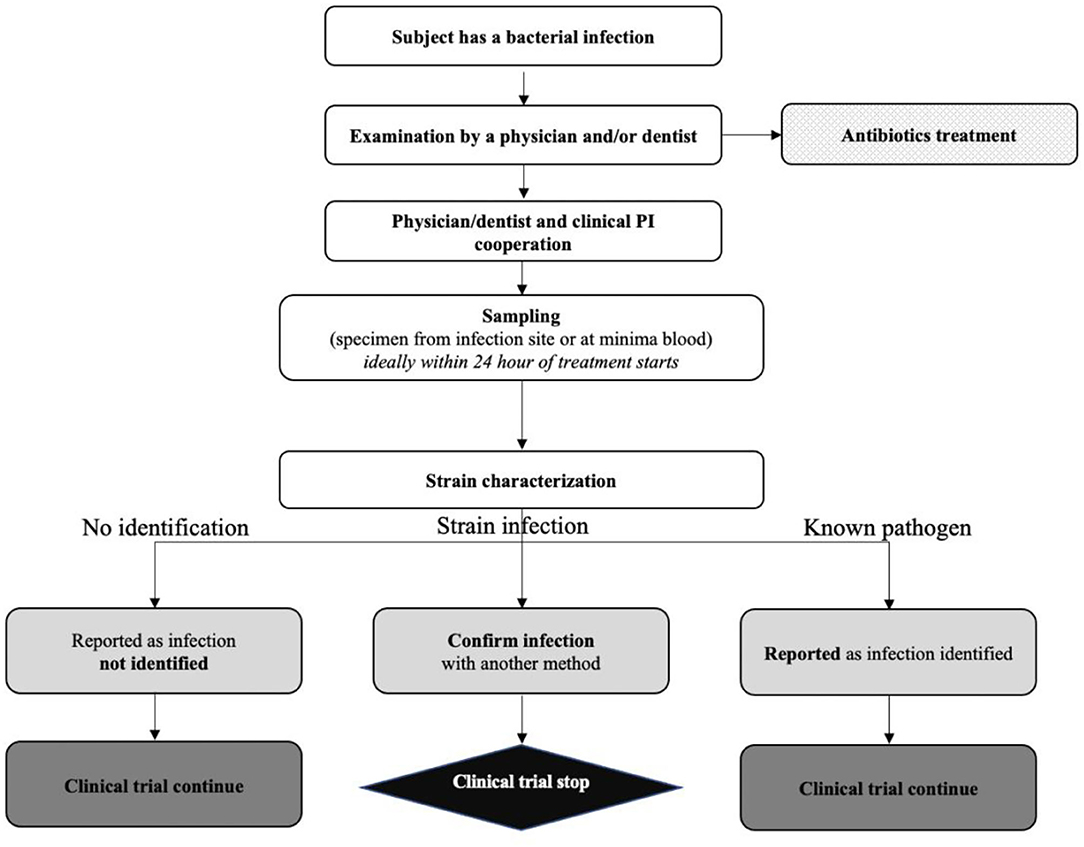
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA
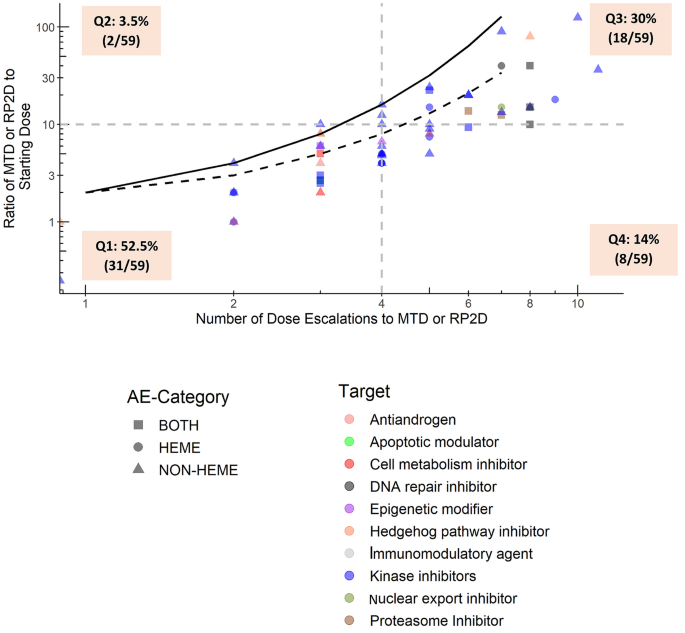
Starting dose selection and dose escalation for oncology small molecule first-in-patient trials: learnings from a survey of FDA-approved drugs | SpringerLink
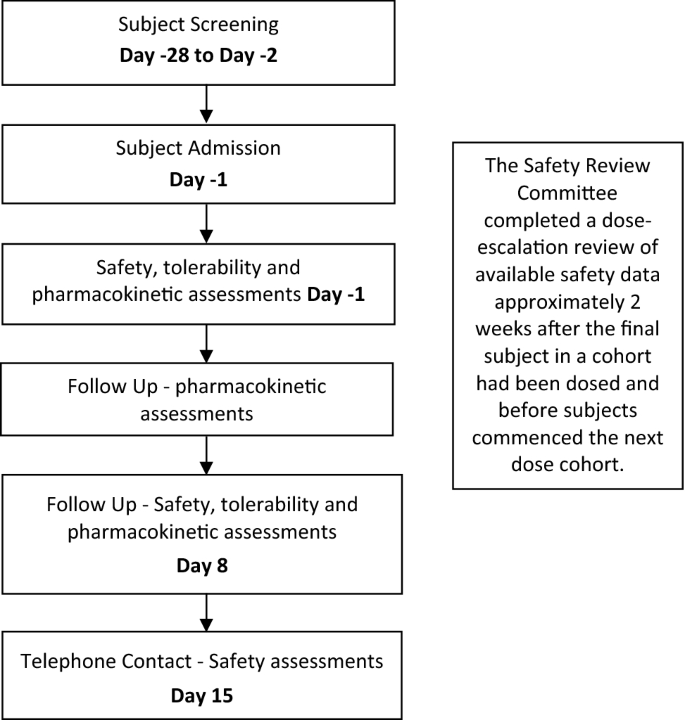
A Phase 1, Randomised, Placebo-Controlled, Dose Escalation Study to Investigate the Safety, Tolerability and Pharmacokinetics of Cannabidiol in Fed Healthy Volunteers | SpringerLink
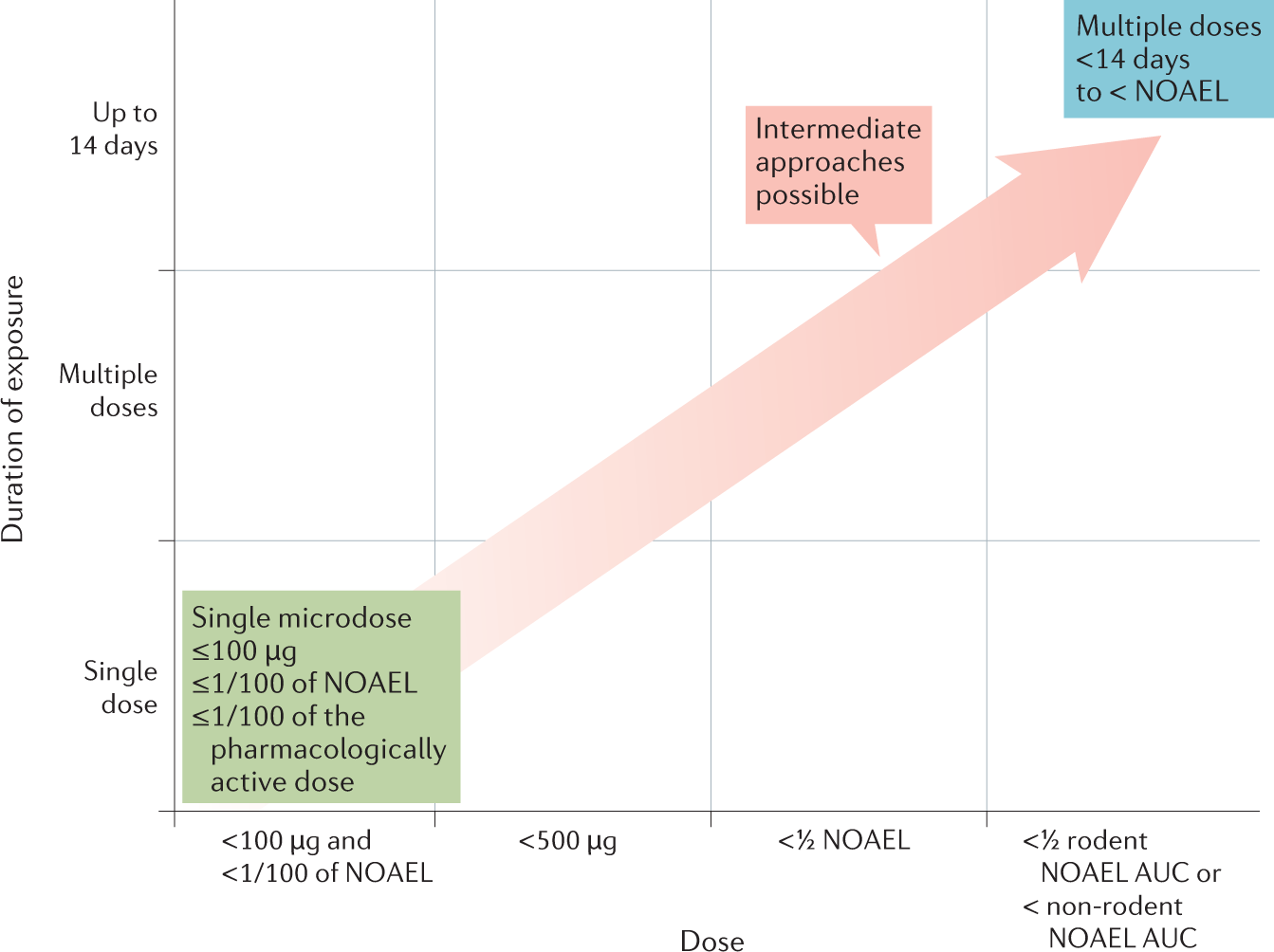
Phase 0/microdosing approaches: time for mainstream application in drug development? | Nature Reviews Drug Discovery

1 US FDA general guide for FIH dose selection for a cytotoxic agent and... | Download Scientific Diagram

Exposure driven dose escalation design with overdose control: Concept and first real life experience in an oncology phase I trial - ScienceDirect

Immune checkpoint inhibitor-based combinations: is dose escalation mandatory for phase I trials? - Annals of Oncology

FDA draft guidance aims to expedite first-in-human clinical trials for oncology drugs and biologics - Pearl Pathways

Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library