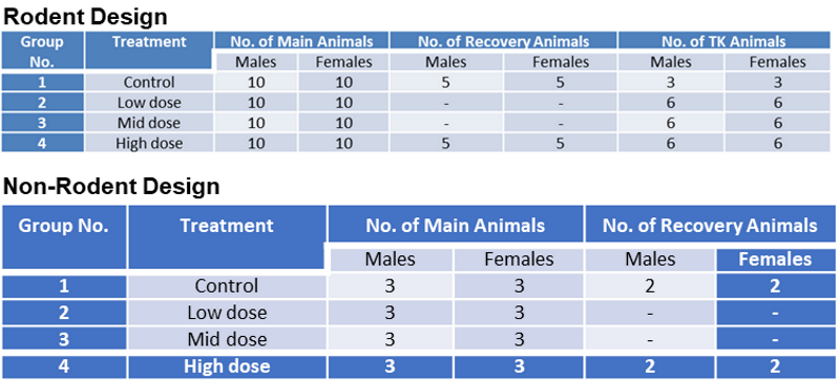
In Silico Models for Repeated-Dose Toxicity (RDT): Prediction of the No Observed Adverse Effect Level (NOAEL) and Lowest Observed Adverse Effect Level (LOAEL) for Drugs | SpringerLink

Molecules | Free Full-Text | Single, 14-Day, and 13-Week Repeated Dose Toxicity Studies of Daily Oral Gelidium elegans Extract Administration to Rats
Evaluation of 90 day repeated dose oral toxicity and reproductive/developmental toxicity of 3'-hydroxypterostilbene in experimental animals | PLOS ONE
Preliminary Assessment of Acute and 28-Day Repeated Dose Oral Toxicity of a Newly Developed Herbal Mixture on Experimental Anima
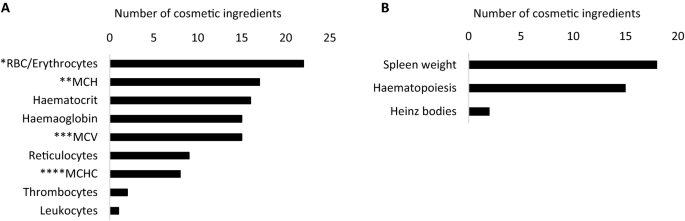
Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019 | SpringerLink
Description of prototype modes-of-action related to repeated dose toxicity - Publications Office of the EU

Foods | Free Full-Text | Single and Repeated Dose 28-Day and 13-Week Toxicity Studies of Oil Prepared from the Internal Organs of the Japanese Giant Scallop (Patinopecten yessoensis) in Mice

Safety evaluation of aqueous extracts of Sanghuangporus vaninii fruiting body in Sprague–Dawley rats - Huo - 2020 - Food Science & Nutrition - Wiley Online Library

Analysis of repeated dose toxicity studies. The study protocols and... | Download Scientific Diagram

Acute and repeated doses (28 days) oral toxicity study of Vicenin-1, a flavonoid glycoside isolated from fenugreek seeds in laboratory mice - ScienceDirect
![PDF] Thesaurus for histopathological findings in publically available reports of repeated-dose oral toxicity studies in rats for 156 chemicals. | Semantic Scholar PDF] Thesaurus for histopathological findings in publically available reports of repeated-dose oral toxicity studies in rats for 156 chemicals. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b71f8ced3d604b24ef24fee271c2ea58800a2f3c/2-Table1-1.png)
PDF] Thesaurus for histopathological findings in publically available reports of repeated-dose oral toxicity studies in rats for 156 chemicals. | Semantic Scholar

Identification of repeated dose toxicity studies described in 88 safety... | Download Scientific Diagram
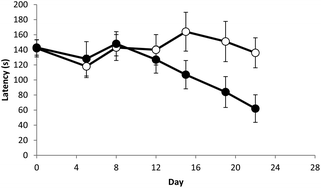
Functional assessments in repeat-dose toxicity studies: the art of the possible - Toxicology Research (RSC Publishing)

Read-across of 90-day rodent repeated-dose toxicity: A case study for selected simple aryl alcohol alkyl carboxylic acid esters - ScienceDirect

Repeated-doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in Wistar rats - ScienceDirect
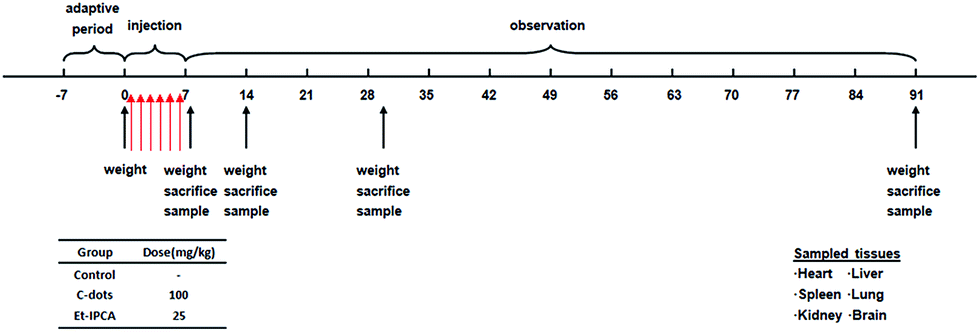
Single and repeated dose toxicity of citric acid-based carbon dots and a derivative in mice - RSC Advances (RSC Publishing) DOI:10.1039/C5RA18391J